A proton has a mass of 16726 x 10-24grams. The protons are located inside the nucleus and have a positive charge.
Subatomic Particles Atomic Number And Atomic Mass Ppt Video Online Download
Ants would be far too big to represent as the electrons.

. Each isotope of a given element has the same atomic number but a different mass number A which is the sum of the numbers of protons and neutrons. - use atomic mass atomic number and charge to identify neutral atoms ions and isotopes. One of the descriptions describes two particles.
Masses for the three subatomic particles can be expressed in amu atomic mass units or grams. The electrons are located outside the nucleus and have a negative charge. Identify which subatomic particles match each of these descriptions.
List the subatomic particles and describe their relative masses charges and positions in the atom Atom. Imagine the atom as the size of a professional baseball stadium in 3D. - analyze the structure of the.
Properties of the subatomic particles are given in the table below. Like other charged particles the difference in rest mass value fits within. An electron has a relative charge of -1602.
E3radg8 and 1 more users found this answer helpful. Subatomic particles present in the nucleus are known as nucleons eg. The relative masses of atoms are reported using the atomic mass unit amu which is defined as one-twelfth of the mass of one atom of carbon-12 with 6 protons 6 neutrons and 6 electrons.
- illustrate the structure of the atom by using the Bohr model including the charge relative mass and location of the sub-atomic particles. Atoms contain three sub-atomic particles called protons neutrons. K0 has a PDG rest mass value of 04976 GeV.
Atom is made up of three subatomic particles. The atomic mass unit is different from other units of measurement because it is developed by comparing the mass of atoms to the mass of Carbon-12 an atom of carbon. In contrast the electron has a negligible mass of 0005 amu.
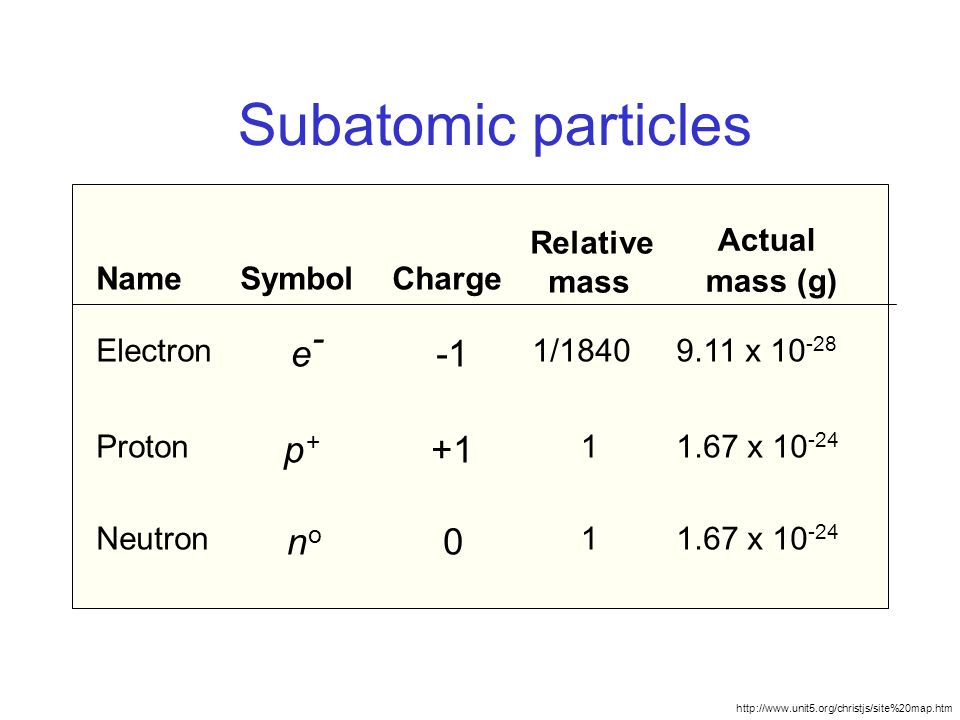
Protons electrons and neutrons. Both neutrons and protons are assigned as having masses of 1 amu each. Properties of Subatomic Particles Particle Charge Relative mass Proton 160 x 10-19 C 1 amu Electron -160 x 10-19 C 0 amu 11840 amu Neutron neutral 1 amu.
Provide students with information regarding the relative mass of the proton and the electron. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. Then tell students that the distance of the electron from the.
The mass of subatomic particles is measured in atomic mass units amu. The relative mass of a proton is 1 and a particle with a relative mass smaller than 1 has less mass. The building blocks of elements Proton.
A proton has a mass approximately 1836 times greater than the mass of the electron but the masses of protons and neutrons differ less than one percent. Protons are practically the same size as neutrons and both are much larger than electrons. That an atom is 999999 empty space.
The mass of a proton is about 1800 times greater than the mass of an electron. The relative charge of protons is 1 the relative mass of a proton is 1 and a proton is located in the center of the atomnucleus. K-and K have a PDG rest mass value of 04937 GeV.
The largest subatomic particle by mass is the proton. The relative charge of the neutron is 0 the relative mass of a neutron is 1 and the neutron is located in the center of atomsnucleus along with protons. The size of the nucleus would be about the size of a baseball in proportion.
The protons and neutrons are found in the nucleus at the centre of the atom. The proton has a mass of 1007 amu the neutron has a mass of 1 amu. The subatomic particles of atoms are Protons Neutrons.
The mass of electrons is very small compared to protons and neutrons. From the table the mass of an electron is so small that it is assigned a value of zero. A proton has a relative charge of 1602 x 10-19 Coulomb and a mass of 1672 x 10-24 gram.
There are three kaons K. The rest masses are slightly different which is explained below. Subatomic particle Relative mass Relative charge Proton 1 1 Neutron 1 0 Electron 00005 -1.
Atoms are uncharged species due to the presence of an equal number of protons. Subatomic particle Relative mass Relative charge. Atom is defined as the smallest unit of a matter.
Protons and neutrons are almost identical in mass whereas electrons are much smaller than the other two. Make sure to include both particles it describes. Two are charged particles K-and K and one is a neutral particle K0.
The amu you see attached to the exact mass values is called the atomic mass unit. Answer 1 of 5. Positive charge found in nucleus 1008 amu.
Atomic Structure - describe the characteristics of protons neutrons and electrons in terms of location charge and mass. If I tell you that the lithium nucleus has a charge of 3 you know it has 3 protons. Actually I found several comparisons that all seem a little different.
Description Particles have a relative charge of 1 have a relative charge of -1 have no charge located in the nucleus of an atom have a much lower mass than the other two types of. What is the rationale for using the relative masses and the relative charges of subatomic particles rather than their absolute masses and charges. The neutrons are located inside the nucleus and have no charge.
For simplicity we will use the amu unit for the three subatomics.

Subatomic Particles Atomic Number And Atomic Mass Ppt Download
0 Comments